Formula for an Ionic Compound Made of Magnesium and Sulfur
Magnesium sulfide represented by the chemical formula MgS that bears the IUPAC name sulfanylidenemagnesium is a white crystalline inorganic compound that is moderately soluble in water and acid. The formula for aluminum oxide is Al2O3.

What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Lisbdnet Com
Aluminium oxide contains Al 3 and O 2 ions.

. In order to bond ionically the charges must be equal. It is an ionic compound of magnesium and sulfur. 30 What is the formula for an ionic compound made of aluminum and oxygen.
- 4155031 ambrose7616 ambrose7616 06212017 Chemistry High School answered What is the formula for an ionic compound made of magnesium and sulfur. What is the formula for an ionic compound made of magnesium and sulfur A MgS B from CHEM 113 at Northwest Missouri State University. The formula for an ionic compound must contain the same number of positive and negative charges.
The product is magnesium sulfide with formula MgS. Is an empirical formula. What is the formula for an ionic compound made of magnesium and sulfur.
What is the formula for the ionic compound of magnesium and sulfur. O MgS MgS2 Mg2S Mg2S3 Onone of the above. What is the formula for an ionic compound made of magnesium and sulfur group of answer choices.
The molecule is formed by one barium cation Ba 2 and one sulfide anion S 2. The electrostatic force of attraction will then bring the magnesium cations and the sulfur anions. MSG is made of a positive sodium ion Na and a negative glutamate ion which has the molecular formula C5H8NO4.
The two ions are bound trough an ionic bond. This is three positive charges and two negative charges. What is the ionic formula for magnesium and sulfur.
What is the formula for an ionic compound made of magnesium and sulfur. It is an ionic compound of magnesium and sulfur. Magnesium sulfide represented by the chemical formula MgS that bears the IUPAC name sulfanylidenemagnesium is a white crystalline inorganic compound that is moderately soluble in water and acid.
Polar water interacts with these oppositely charged ions to dissolve the Epsom salt. It will take one -2 sulfide ion to balance one 2 magnesium ion forming a magnesium sulfide molecule of MgS. Therefore the proper formula for this ionic compound is MgO.
Aluminum Al has 3 charge and that of oxygen O is -2. The barium sulfide chemical formula is BaS. What is the formula for an ionic compound made of magnesium and sulfur.
MgS Mg2S3 Mg2S MgS2 none of the above. What is the formula of a substance made up of al3 ions and o2 ions. Epsom salt is an ionic compound.
MgS MgS_2 Mg_2S Mg_2S_ What are the coefficients for the following reaction when it is properly balanced. A MgS b MgS2 c Mg2S d Mg2S3 e None of the above. So that the charges are balanced and it is neutral overall.
Chemistry questions and answers. Magnesium Sulfide has a formula of MgS. Click to see full answer.
To write the empirical formula for an ionic compound we use the periodic table to identify the metallic. What are the valencies of. B Al 2 O 3.
What is the formula for an ionic compound of magnesium and sulfur. It will take one -2 sulfide ion to balance one 2 magnesium ion forming a magnesium sulfide molecule of MgS. In order to bond ionically the charges must be equal and opposite.
In order to bond ionically the charges must be equal and opposite. The Lewis theory predicts that the formula for a compound of magnesium and sulfur is MgS2. Although both of these ions have higher charges than the ions in lithium bromide they still balance each other in a one-to-one ratio.
1 See answer Advertisement Advertisement ambrose7616 is waiting for your help. Magnesium Sulfide has a formula of MgS. In order to bond ionically the charges must be equal and opposite.
I hope this was helpful. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. After crisscross the formula formed will be Al 2 O 3.
Working out a formula. What is the formula for an ionic compound made of magnesium and sulfur. A AlO 2 B Al 2 O 3 C Al 3 O 2 D AlO E none of the above.
The electrostatic force of attraction will then bring the magnesium cations and the sulfur anions together an ionic bond is formed. How do you write the formula for barium nitride. The molar mass is 16939 gmol.
Therefore according to this B is the correct option. What is the formula for an ionic compound made of magnesium and sulfur. Add your answer and earn points.
What is the formula for an ionic compound made of barium and sulfur. A a Mg2S2 Mg2S2 MgS a a. 4 rows Magnesium Sulfide has a formula of MgS.
Magnesium Sulfide has a formula of MgS. When an ionic compound is formed from magnesium and oxygen the magnesium ion has a 2 charge and the oxygen atom has a 2 charge. There is a positive magnesium ion Mg2 and a negative sulfate ion SO42.
To make the number of charges the same we need two Al 3 ions and three O 2 ions. So the formula is Al 2 O.

What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Lisbdnet Com

Ionic Bonding In Magnesium Sulfide Mgs Youtube

Ionic Bonding In Magnesium Sulfide Mgs Youtube

What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Lisbdnet Com

Magnesium Sulfide Facts Formula Properties Uses

Solved What Is The Formula For An Ionic Compound Made Of Chegg Com

What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Lisbdnet Com

What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Lisbdnet Com
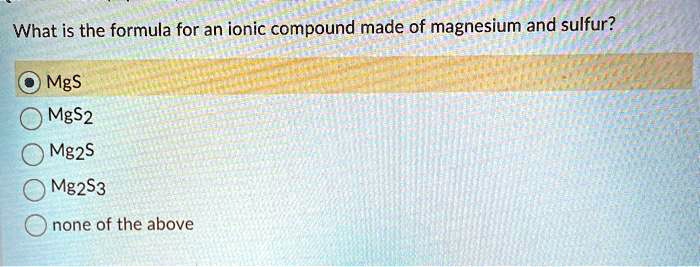
Solved What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Mgs Mgs2 Mg2s Mg2s3 None Of The Above
Komentar
Posting Komentar